The safety, quality, and efficacy of products, especially food, pharmaceuticals, cosmetics, and medical devices, are more critical than ever. One regulatory framework that ensures the integrity of these products is GMP: Good Manufacturing Practice regulations.
These regulations are a cornerstone of quality assurance, helping manufacturers maintain consistent standards across batches, facilities, and even international borders.

What is GMP?
GMP stands for Good Manufacturing Practice, a system that ensures products are consistently produced and controlled according to quality standards. It is designed to minimise risks involved in production that cannot be eliminated through final product testing. These regulations are enforced by various national and international bodies, including the U.S. FDA, European Medicines Agency (EMA), and WHO.
The Origin and Evolution of GMP Regulations
The history of GMP good manufacturing practice regulations dates back to the mid-20th century. In the United States, the Kefauver-Harris Amendments of 1962 laid the groundwork for stricter drug regulation following the thalidomide tragedy. Over time, these practices evolved and expanded globally. Today, GMP is recognised as a universal standard for manufacturing quality, adopted across industries and borders.
Core Principles of GMP
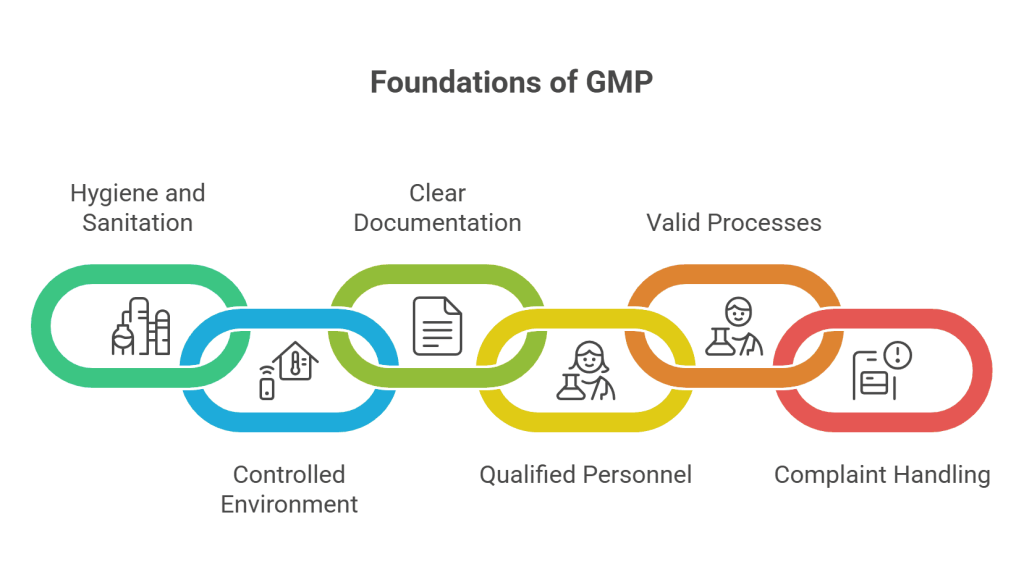
The framework of GMP good manufacturing practice regulations is built on several key Principles of GMP:
- Hygiene and Sanitation: Facilities must be clean and maintained to avoid contamination.
- Controlled Environment: Air, temperature, humidity, and pressure must be regulated to ensure product integrity.
- Clear Documentation: Every step in the manufacturing process must be documented to ensure traceability.
- Qualified Personnel: Staff must be trained, competent, and responsible for upholding GMP standards.
- Valid Processes: Manufacturing methods must be thoroughly tested and validated to ensure consistent and reliable results.
- Complaint Handling and Recall Systems: Mechanisms must be in place to handle defective products and recall them from the market.
These principles ensure that every product is manufactured with safety, quality, and compliance in mind.
Industries That Require Good Manufacturing Practice GMP Regulations
While pharmaceuticals are the most closely associated with GMP, many industries fall under the scope of GMP good manufacturing practice regulations, including:
- Food and Beverage
- Nutraceuticals and Dietary Supplements
- Cosmetics and Personal Care Products
- Biotechnology and Medical Devices
For each of these sectors, failure to comply with GMP can result in severe consequences—ranging from product recalls to loss of licenses or legal action.
Key Components of GMP Systems
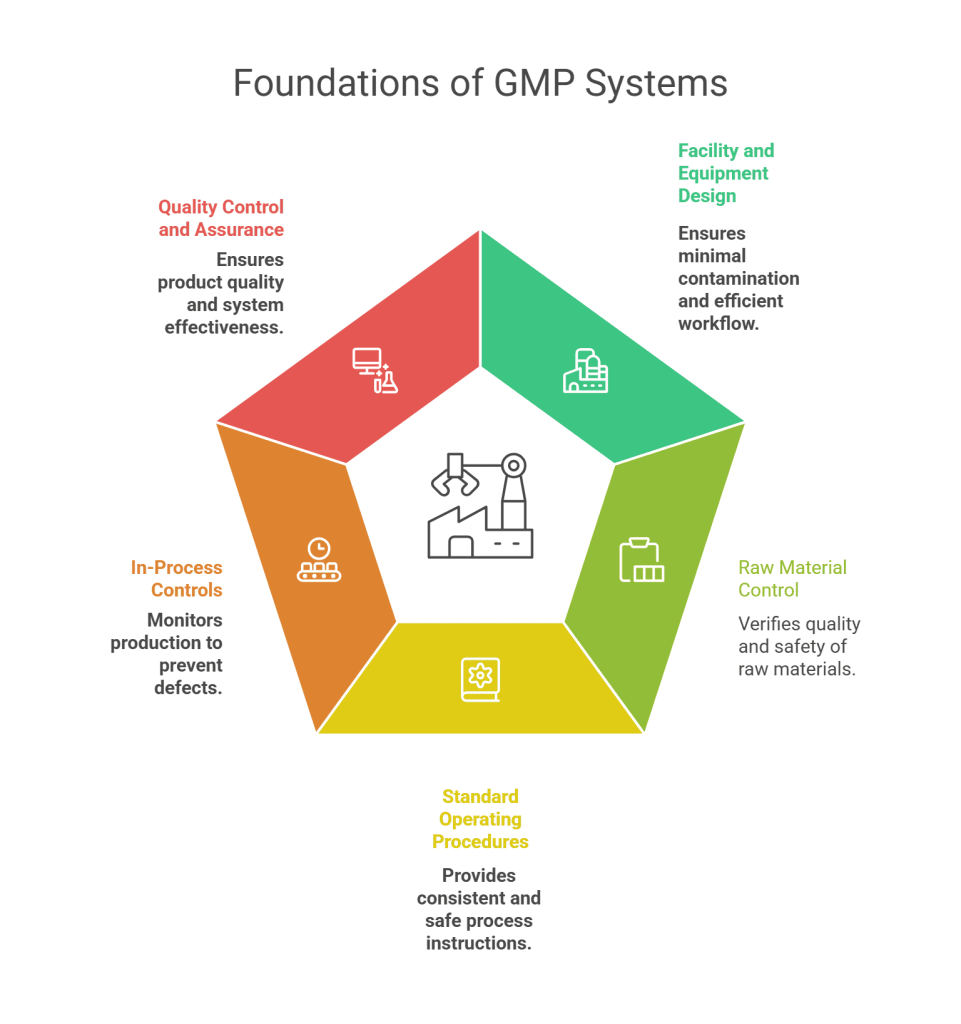
1. Facility and Equipment Design
GMP regulations mandate that facilities should be designed to minimize contamination, allow proper cleaning and maintenance, and support logical workflow. Equipment must be properly maintained, calibrated, and validated.
2. Raw Material Control
All raw materials used in production must be verified for quality and safety. This includes maintaining supplier qualifications, incoming inspection, and material traceability systems.
3. Standard Operating Procedures (SOPs)
SOPs are at the heart of GMP good manufacturing practice regulations. These detailed, written instructions ensure that every step of the process is performed consistently and safely.
4. In-Process Controls and Testing
Real-time monitoring during manufacturing ensures that the product meets its specifications. In-process controls help identify deviations early and prevent defective products from reaching the market.
5. Quality Control and Quality Assurance
Quality Control (QC) involves product testing, while Quality Assurance (QA) ensures that the systems and processes work correctly. Together, they form a strong foundation for compliance with GMP standards.
Common GMP Violations and How to Avoid Them
Even well-established companies occasionally slip up in maintaining GMP good manufacturing practice regulations. Some common violations include:
- Inadequate documentation
- Poor maintenance of equipment
- Lack of personnel training
- Cross-contamination risks
- Failure to investigate deviations
To prevent these violations, companies should foster a compliance culture, invest in regular audits, and train their employees continually.
GMP Certification and Audits
Getting certified in GMP is often not mandatory but highly recommended, especially when exporting products to countries with stringent regulatory requirements. GMP audits are conducted by internal teams or third-party organizations to assess compliance. These audits evaluate whether the systems, processes, and personnel meet the expectations set by GMP good manufacturing practice regulations.
International Harmonization of GMP Standards
As global trade grows, harmonization of GMP standards has become crucial. Organizations like ICH (International Council for Harmonization) and PIC/S (Pharmaceutical Inspection Co-operation Scheme) work to align GMP practices globally.
Harmonized standards help multinational manufacturers ensure product quality across different jurisdictions and reduce the risk of regulatory conflicts.
The Role of Technology in GMP Compliance
Modern manufacturing has benefited greatly from digitalization and automation. Electronic Batch Records (EBRs), Manufacturing Execution Systems (MES), and automated quality control systems have improved traceability and reduced human error. These technologies make it easier to comply with GMP good manufacturing practice regulations while increasing efficiency and scalability.
Future Trends in GMP Regulations
As the industry evolves, so do the regulations. Key future trends include:
- Data Integrity: Ensuring that electronic data is complete, consistent, and accurate.
- Environmental Sustainability: Regulatory agencies may begin factoring in green manufacturing practices.
- Risk-Based Approaches: More emphasis on identifying and managing risks proactively rather than reactively.
- Remote Audits: Virtual inspections are becoming more common post-COVID-19.
These changes reflect a shift toward more dynamic, responsive, and tech-driven compliance models within the framework of GMP good manufacturing practice regulations.
FAQs on GMP: Good Manufacturing Practice Regulations
1. What is the main purpose of GMP?
The primary goal of GMP is to ensure that products are consistently produced and controlled to quality standards, minimizing risks such as contamination, mix-ups, and deviations.
2. Is GMP mandatory for all manufacturers?
GMP compliance is mandatory for regulated industries like pharmaceuticals, food, and cosmetics. Even in non-regulated industries, voluntary compliance is often necessary for credibility and market access.
3. What happens during a GMP audit?
A GMP audit involves reviewing documentation, inspecting facilities, and interviewing personnel to assess whether a company adheres to GMP standards. Findings can result in observations, warnings, or even legal action if non-compliance is severe.
4. How often are GMP audits conducted?
The frequency varies based on the industry and the company’s history. Some may be audited annually, while others are audited every two or three years. Regulatory bodies can also conduct surprise inspections.
5. Can small businesses comply with GMP?
Yes, small manufacturers can and should comply with GMP good manufacturing practice regulations. The scope of implementation may be smaller, but the fundamental principles remain the same.
6. What’s the difference between GMP and cGMP?
cGMP stands for “current Good Manufacturing Practice.” It emphasizes that regulations are not static and should evolve with technological and scientific advances.
7. Does GMP compliance ensure product safety?
While GMP greatly reduces the risks associated with manufacturing, it does not guarantee product safety. However, it provides a robust system that enhances overall quality and integrity.
Conclusion
Whether you’re a pharmaceutical giant, a food startup, or a cosmetics manufacturer, understanding and implementing GMP good manufacturing practice regulations is essential for sustainable success. These regulations form the backbone of modern manufacturing quality systems, helping companies meet legal requirements, satisfy customers, and build trust in their products.
As technologies evolve and consumer expectations rise, GMP compliance will continue to be a dynamic, integral part of responsible manufacturing. Traceability is a crucial factor in compliance and manufacturing. Contact Qodenext for end-to-end visibility.