The security of pharmaceuticals relies on accurate tracking. The active pharmaceutical ingredients (APIs) are the building blocks of all medications. They need an ombudsman for their journey from manufacturer to patient. Tracking is critical in the face of contamination risks, counterfeiting threats and regulatory compliance. If the tracing systems are deficient, patient safety is endangered. APIs need to be very closely watched at each stage of the supply chain. This guarantees quality, authenticity and responsibility from the beginning to the end of the pharmaceutical industry.
Moreover, if you are a member of the pharmaceutical supply chain, the presence of low-quality counterfeit drugs can hurt your trade and damage your reputation. Above all, it reduces the public’s faith in the overall healthcare system.

To combat this concern, the Government of India has devised an API (Active Pharmaceutical Ingredients) Traceability Regulation outlining track-and-trace requirements for APIs.
Along those lines, let us look into the bigger picture.
Understanding APIs: What is an Active Pharmaceutical Ingredient?
Active Pharmaceutical Ingredient (API) indicates the presence of the biologically active raw material in the drug that leads to the therapeutic effects. In essence, the active pharmaceutical ingredient definition tells us what makes the medicine work.
Active Pharmaceutical Ingredient (API) List :
Paracetamol in painkillers
Ibuprofen in anti-inflammatories
Amoxicillin in antibiotics
An active pharmaceutical ingredient example like aspirin can be used to treat many different illnesses. Another active pharmaceutical ingredient list example is insulin for diabetes management.
Key Points:
Produces desired medical effects
Potency of the drug
Needs to be tightly controlled in quality
Types of Active Pharmaceutical Ingredients (APIs)
APIs or Active Pharmaceutical Ingredients are the ingredients present in drugs that deliver the envisioned result. In the pharmaceutical industry, APIs are the biologically active parts of a drug contained in medicines like capsules, tablets, creams, injections, etc. As APIs are very strong, small quantities are enough to deliver the desired result.
There are two primary types of Active Pharmaceutical Ingredients (API)s:
- Synthetic Chemical API: Also known as small molecules, the APIs hold a sizeable part of the modern commercial pharmaceutical trade. Some common examples include diphenhydramine, aspirin, etc.
- Natural Chemical API: These APIs are used to make biologics. These are increasingly popular in the present pharma market. Some common examples are Remicade, Humira, Rituxan, etc.
Now, imagine counterfeits of these vital drugs and their impact on public health. Considering the consequences, the Indian Government has taken reasonable measures to battle counterfeit drugs.
Why Is Traceability Integral to API Manufacturing?
Several reasons justify the need for supply chain traceability for APIs. For manufacturers, traceability guarantees safety and quality. If a problem occurs, the source can be identified quickly, regardless of the production stage, thus preventing patients from getting their hands on inadequate or unsafe products.
However, the benefits are certainly not restricted to manufacturers. Every supply chain member contributes towards improved supply chain visibility by adhering to traceability requirements.
One of the major benefits of end-to-end traceability is that it helps supply chain members streamline inventory management, which ensures fewer delays and reduces losses.
What Are the Active Pharmaceutical Ingredients (API) Traceability Regulations to Follow?
The Indian Government has announced that all Active Pharmaceutical Ingredients (APIs) packages should have a unique QR code or identity from January 1, 2023.
This reform is designed to ensure falsified API batches are easily identified. Most importantly, it aims to track tampered or substandard products and send them back to manufacturers.
According to the API Traceability Regulation, active ingredients manufactured or imported in India should bear a QR (Quick Response) Code on the label, specifically at every level of packaging.
When the QR code is present, it will permit supply chain members to read through product info simply by scanning the QR code. Furthermore, it would also allow stakeholders to track as well as trace throughout the supply chain journey.
What Does the QR Code Contain?
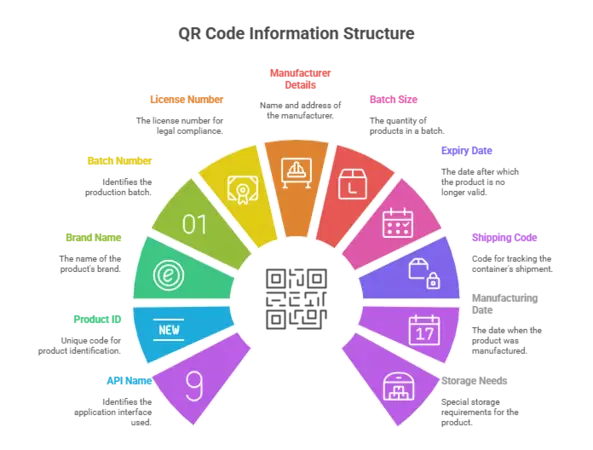
On each pack level, the QR code would include an exclusive product identifier, API name, manufacturer name and address, batch information, manufacturing and expiry date, and import license numbers. It also links to the national database having pricing data, which is exclusively in control of the National Pharmaceutical Pricing Authority.
The Drug and Technical Advisory Board (DTAB) of India approved the mandatory QR code on APIs proposal in June 2019, highlighting that it would apply to domestically produced as well as imported ingredients and impact over 2500 APIs used for manufacturing pharmaceutical products in India.
Briefly, the QR Code contains vital information like:
- API name
- Exclusive product identification code
- Brand name
- Batch number
- License number
- Manufacturer name and address
- Batch size
- Expiry date or retesting date
- Serial shipping code of the container
- Manufacturing date
- Special storage needs (if applicable)
How Does API Traceability Benefit the Pharmaceutical Industry?
With traceability and serialization, pharmaceutical supply chains can keep a tab on all the APIs traversing through supply chains. It would help stakeholders identify unauthenticated and potentially counterfeit drugs and enable swift product recall and investigations.
Most importantly, the foreseen online system would assist manufacturers and traders in using and generating both secondary-level and tertiary-level coded data, allowing seamless aggregation of all pharmaceutical products.
In essence, the end-to-end tracking and tracing system allows manufacturers and other supply chain members, like re-packagers and dispensers, to distribute efficiently. Moreover, as data is logged in the centralized system, the inventory management process becomes hassle-free.
The Final Word
With API regulation, the Government of India has taken a giant step toward establishing a highly sustainable pharmaceutical supply chain. Besides, India has two established regulatory efforts towards finished medicine traceability – one that targets exports having some serious teething problems that lead to delayed implementation, and the other related to domestic medicines struggling to speed up.
The regulations are directed towards helping track and trace at every stage and creating a more robust supply chain for the pharmaceutical industry that is free from any low-quality or counterfeit products.
QodeNext offers the right solution to supply chain traceability for all products and commodities. Leverage the critical features to give your business the much-needed boost in the public interest. Contact us today!
Frequently Asked Questions(FAQ’s)
1. What is active pharmaceutical ingredient production?
APIs are produced by chemical synthesis , fermentation or extraction. Needs to be purified, tested for quality and hold to stringent regulatory requirements during manufacturing.
2. What is active pharmaceutical ingredient?
The compound in medication having a therapeutic effect in a treatment. It is the primary ingredient that helps cure a disease or medical condition.
3. How are APIs prepared?
Chemical synthesis, biological fermentation, or natural source extraction. All batches are purified, tested, and quality assured to the letter.
4. What IS THE difference between API and excipient?
API has therapeutic effect; excipients are inert material. Excipients help with delivery, stability and absorption, not to treat disease.
5. Which help the major types of API?
Synthetic (made by chemical process), biological (living organism), natural (plant/ animal extracts). Each species of medicine these has specific therapeutic intent with specific methods of production.
6. What Are Generic APIs?
These are the products which are pharmaceutically equivalent with the branded APIs once the patent expiry. They have the same active ingredients, so the safety, effectiveness and quality are the same even though the costs are lower.